Citing sero-survey results and lack of clarity around the efficacy of the vaccines, experts are wondering if only the truly vulnerable need them
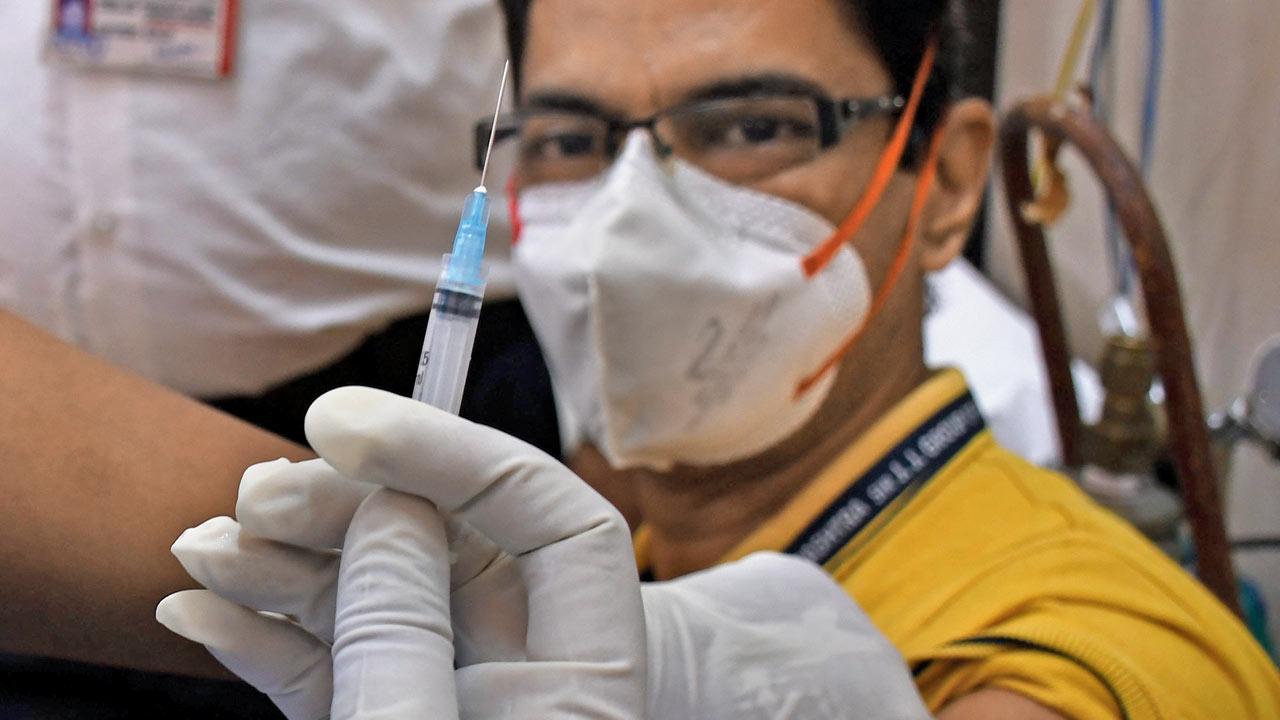
The vaccine being administered to a health worker at JJ Hospital on Saturday. Pics/Ashish Raje
The glitches in CoWIN may have delayed the vaccination drive in Mumbai, but as per a recent sero-survey, more than 50 per cent of the Indian population has developed herd immunity and concerns are being raised about the need to vaccinate the entire country, due to this. Sanjiv Agarwal, founder of Good Governance India Foundation, a public policy think tank, working closely with globally renowned health care experts, says the vaccine is only for those still vulnerable.
ADVERTISEMENT
Health workers at Nair Hospital on Saturday
Agarwal said, “India is probably the only country that is expected to develop herd immunity at a fast pace while keeping the death rates very low, and yet has vaccines rolling out. The natural immunity attained due to COVID antibodies may not be lifelong, but is quite effective and lasting. Even those who suggest vaccination, admit that it is not clear as to how long the vaccination-induced immunity will last. Moreover, vaccines that are based on inactivated viruses (like Covaxin) possibly cannot impart better antibodies than the natural ones. Hence, it cannot be scientifically argued that those who have already developed natural immunity to COVID-19 must also be administered a vaccine on an emergency basis.”
‘Vaccinate those vulnerable’
Agarwal continued, “It would be too costly, time consuming, and perhaps unethical to force the entire population of India to take the vaccine, when it seems to have already achieved a high level of natural immunity, as admitted by policymakers. Vaccination can be reserved for vulnerable groups who may not have developed natural immunity yet.”
The daily deaths due to COVID have come down to approximately 200 from the peak of around 1,000, he said. This is a small number if you consider that more than 26,000 people die in India every day due to different diseases for example about 2,000 people die from diarrhoea and 1,200 from tuberculosis, every day, he said. “The drastic decrease in daily deaths is due to herd immunity as a result of widespread infection rather than lockdowns or other control measures. The lockdown was unnecessary and possibly ruined many people, destroyed the economy, while nature was at work spreading the infection at a fast pace - a blessing in disguise because that is how herd immunity is built,” Agarwal said.
“A swift, focused protection strategy to administer the vaccine, which targets only the most vulnerable, with a choice to test whether they have developed natural immunity, will be the best course of action,” Agarwal concluded.
‘Issues with consent’
Senior legal brains have raised concerns about the Covaxin maker coming up with their consent form stating it is under trial phase, while the maker of Covishield has not issued any such consent, underlining the need to bring more transparency in the entire vaccine programme.
Advocate Prasanna S said, “There are several issues with the consent notice of BBIL (M/s Bharat Biotech International Limited). Consent, in law, has to be free and informed. For true informed consent, this notice has to be in multiple languages and the signature is to be obtained against the notice that has the subject’s language. Or the subject has to be read out the contents in a language which he understands.”
“It is also not clear if the consent notice is from the ministry of health or BBIL. The notice bears no signature of the issuing authority, which makes it unclear if it is a legally enforceable form. Further, it is not clear if the liability of BBIL or the government of India is limited and to what extent, in case of a compensation claim,” said Prasanna.
Solicitor Stuti Galiya said, “The Drugs and Cosmetics Act, 1940 includes guidelines for the conduct of clinical trials, to ensure that patients and volunteers participate in them only after a complete understanding of the investigative study. It is the duty of the physicians to apprise the trial subjects about the experiments in detail. The consequences of being a party to the trial should be made known to the patient; consent documents should be approved by the ethics committee and submitted to the Central Licensing Authority.”
Galiya added, “The regulations also mandate that the concerned hospital is required to maintain an audio-video recording of the informed consent process in case of vulnerable subjects in clinical trials of a new chemical entity. As COVID-19 falls under clinical trials of a new chemical entity, there should have been an audio-video recording of the informed consent process. It is important that the process as prescribed under the Act to conduct clinical trials is duly followed, to ensure that vaccine administration done in haste does not fall short of the legal requirements.”
 Subscribe today by clicking the link and stay updated with the latest news!" Click here!
Subscribe today by clicking the link and stay updated with the latest news!" Click here!






